“Time is of the essence.”
The statement, made by Andrew Kline of the National Cannabis Industry Association (NCIA), encapsulates the views of many people who attended a recent public hearing hosted by FDA on cannabidiol (CBD) products.
During the May 31 hearing in Silver Spring, Maryland, several speakers urged FDA to provide clarity to a confused marketplace and do so swiftly.
“We strongly recommend that FDA act quickly to clarify the regulatory environment because there is significant confusion in the market,” Kline said.
FDA is mulling whether to issue regulations that would permit CBD in conventional food and dietary supplements, but its search to answer questions over safety, effectiveness and other issues could lead to a years-long proceeding.
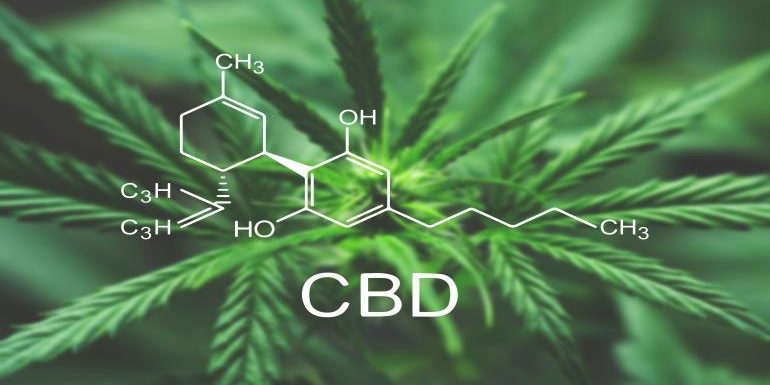
Based on its current interpretation of federal law, FDA has opined CBD cannot be added to conventional food or marketed as a dietary supplement because, in large part, it was first studied as a drug.
“Three to five years, at a minimum for rulemaking, is too long,” Megan Olsen, assistant general counsel of the Council for Responsible Nutrition (CRN), told FDA officials during the public hearing.
The debate over safety, she added, should not hamper FDA from promulgating regulations to allow for the use of CBD in supplements.
“There is already a regulatory framework in place that is proven to ensure the safety of dietary supplements and food—one that will automatically be implemented should FDA develop a regulation permitting CBD use in food and supplements,” Olsen said.
Read The Full Article HERE